Equine Drugs, Medications, and Performance Altering Substances: Their Performance Effects, Detection, and Regulation

Dr. Thomas Tobin, Dr. Julio
Gutierrez, Emily Schwartz,
Dr. Fernanda Camargo, and Charlie Hughes
Equine Pharmacology, Therapeutics and Toxicology Laboratory
The Maxwell H. Gluck Equine Research Center
University of Kentucky
Lexington, KY 40546-0099
Dr. Rodney Eisenberg
Frontier Biopharm
6013 Atwood Drive, Suite 300
Richmond, KY 40475
e-mail: rod@frontierbiopharm.com
Dr. Andreas Lehner
Diagnostic Center for Population and Animal Health
College of Veterinary Medicine
Michigan State University
4125 Beaumont Road
Lansing, MI 48910
Mr. Kent Stirling
Florida Horsemen’s Benevolent and Protective Association
P.O Box 1808
Opa Loca,
FL 33055-0808
Based on a presentation to the Equine Law section
of the Kentucky Bar Association at Keeneland,
Lexington, Kentucky, Oct 21, 2005
(webpage updated Dec 2010)
Table of Contents
1 Summary
2 Background and Definitions
3 History
4 Can Drugs or Medications Influence the Outcome of a
Race?
5 The Introduction of ELISA Testing (1988)
6 Mass Spectral Confirmation
7 Liquid Chromatography/Mass Spectrometry/Mass
Spectrometry
(LC/MS/MS)
8 "Zero Tolerance" Testing
9 Numbers of Medication Molecules: Medication Dosing and Elimination
10 Thresholds, Including "No Effect Thresholds"
(NETs)
11 Withdrawal Time Guidelines
12 Reference Standards
13 Medication Rules
14 The Current Racing Medication Testing Consortium (RMTC)
&
Association of Racing Commissioners
International (ARCI) Rule
15 Further Reading
16 Appendices
1. Summary
Thoroughbred Racing has been testing for drugs and medications since about 1903.
Today, racehorse testing is by far the longest established, broadest in scope and most sensitive drug testing performed on earth. Racehorse testing is also performed within an extremely stringent regulatory context, and my understanding is that many of our constitutional protections as US citizens are inoperative in the racing environment. Racehorse testing is also
remarkably “clean,” as the incidence of deliberate use of performance affecting substances seems to be very small.
There are good reasons for all of the above. It is empirically clear that medications
are highly likely to influence the performance of racing horses, although the scientific evidence for
actual improved performance is much less than overwhelming.
In the mid-nineteen eighties, however,
the use of high potency drugs with clear potential to affect performance
was not particularly well controlled. Following a directive from the
Kentucky State Racing Commission, an interdisciplinary team at the
University of Kentucky worked on adapting ELISA testing to racing
chemistry; this proprietary technology was at that time a major step
towards solving the problem of the abuse of high potency drugs in racing
horses, and these tests are now marketed worldwide out of Lexington (www.neogen.com/forensickits.htm)
One of the lessons that came out of
ELISA testing is that advances in drug detection/testing are research
driven. Once a medication is “called positive”, that is the first
“positive” is called and prosecuted, the rate of use of the
substance drops
dramatically, to close to zero, but not quite zero; it appears that
there are always people ready to try a medication that worked for them,
or for a colleague, or a
rival, in the past.
Overall, the rate at which performance altering
medication violations are reported in racing is extremely small. For
example, from 1995–1999 there were about 3 positives for every 100,000
samples for Association of Racing Commissioners International [ARCI]
Class 1 violations after trace level identifications of dietary and
environmental substances are eliminated. By far, the most common
identifications reported in racing are residual “traces” of well
recognized and widely used therapeutic medications, so called
“tail-ends” of therapeutic medications , and traces of dietary and
environmental substances that also happen to be ARCI substances, for
example trace level identifications of caffeine and other substances
widely used by humans.
The ease with which such “traces” of therapeutic
medications, dietary and environmental substances can be detected using
current testing technology has now clearly led scientists and regulators
away from the old “zero tolerance” approach, which many authorities
now see as outdated, to defined regulatory limits or “thresholds”
for therapeutic medications, endogenous, dietary and environmental
substances.
This
situation was driven in large part by ELISA testing, which allows highly
sensitive detection of trace amounts (tail ends) of therapeutic
medications, environmental and dietary substances. In the nineteen
nineties, following another Kentucky Racing Commission directive, the
University
of
Kentucky
program at The Maxwell H. Gluck Equine Research Center
pioneered the basic research that underpins the evolving and now in
principle very well established concept concerning the use of regulatory
“thresholds” in racing regulation.
More recent challenges include developing effective
regulatory methods for the newer recombinant hormonal products such as
the various human recombinant erythropoietin products and variants
thereof and growth hormones. More recently, a high quality ELISA test
has been made available for human recombinant erythropoietin and racing
chemistry has scored a major scientific breakthrough by developing the
first mass spectral confirmation method to detect use of recombinant
human erythropoietin (rhEPO) in horses or, indeed, in any species.
2. Background and Definitions
There
are at least 30 million known chemical substances and 4,000 or more
prescription medications. Racing regulators in the
United
States
,
therefore, divide drugs and medications into two major groups:
The
largest group of concern to regulators is the
"performance-enhancing substances", whose identification in a
horse is viewed with great regulatory concern. Testing for these
substances usually proceeds at the highest level of sensitivity
possible; so-called "zero-tolerance" testing. About 900 or so
substances are classified by the Association of Racing Commissioners
International (ARCI) Uniform Classification System for Foreign
Substances as potentially performance enhancing in a five class system,
the most complete listing of such substances available anywhere in the
world (http://www.arci.com/druglisting.pdf).
The second and smaller group comprises the "therapeutic
medications", recognized by the American Association of Equine
Practitioners [AAEP] and the Racing Medication and Testing Consortium [RMTC].
There are approximately 50 plus of these medications used
therapeutically in horses in training (Table 1). Since about the year
2000, it has come to be much more generally accepted that we must set
“limitations” on the sensitivity of testing for therapeutic
medications. These limitations are variously called thresholds or
reporting levels, or decision levels (
California
)
apparently depending on the semantic preference of the individual
jurisdiction.
Table 1. Therapeutic Medications Routinely Used and Identified as Necessary by the Veterinary Advisory
Committee — (Racing Medication and Testing Consortium [RMTC] draft list of therapeutic medications, 2005)
|
1. Acepromazine |
17. Dipyrone |
33. Omeprazole |
|
2. Albuterol |
18. Flunixin |
34. Pentoxifylline |
|
3. Aminocaproic Acid |
19. Fluprednisolone |
35. Phenylbutazone |
|
4. Atropine |
20. Fluphenazine |
36. Phenytoin |
|
5. Beclomethasone |
21. Furosemide |
37. Prednisolone |
|
6. Betamethasone |
22. Glycopyrrolate |
38. Prednisone |
|
7. Boldenone |
23. Guaifenesin |
39. Procaine Penicillin |
|
8. Butorphanol |
24. Hydroxyzine |
40. Pyrilamine |
|
9. Cimetidine |
25. Isoflupredone |
41. Ranitidine |
|
10. Clenbuterol |
26. Isoxsuprine |
42. Reserpine |
|
11. Cromolyn |
27. Ketoprofen |
43. Stanozolol |
|
12. Dantrolene |
28. Lidocaine |
44. Testosterone |
|
13. Detomidine |
29. Mepivacaine |
45. Triamcinolone |
|
14. Dexamethasone |
30. Methocarbamol |
46. Trichlomethiazide |
|
15. Diazepam |
31. Methylprednisolone |
|
|
16. DMSO |
32. Nandrolone |
|
3. History
Up to
about 100 years ago there was little concern about the use of medication
in racing horses, and particularly so in
North
America
. The
1800s had seen the purification of cocaine and morphine and availability
of these substances in pure form made the acute stimulant medication of
racing horses a reality. Around the turn-of-the-century (1890-1910), a
number of American trainers went to
Europe
, taking
with them these new “American” medications. As
a group, these trainers were so successful that they became known in
European racing circles as the
"Yankee Alchemists."

Figure 1. Carl Vernet depicts apparently routine
pre-race medication of horses, ca. 1810
| In
the early 1900s the Honorable Mr. George Lambton (Fig. 2), the sartorially
correct and socially prominent leading
English trainer of his time, grew tired of losing races to the
"Yankee Alchemists," as he also soon grew tired of
politely requesting the English Jockey Club to do “something”
about the problem. He therefore purchased some of the American
"medications," and publicly announced that certain horses
in certain races were going to be, well, shall we say
"medicated." These activities rapidly gained the Jockey
Club's attention, and in 1903 the Jockey Club made the medication of a racing horse
an offense against the rules of racing in England. While
the record is silent as to how these medications were to be
detected, the prescribed
punishment was to be "ruled off the turf," a punishment
still in place in parts of the English speaking world.
|

Figure
2.
The Honorable George Lambton |

Figure 3.
Somewhat
farther from home, an American trainer by the name of Jack Keene was also
having a very good run in Russia. Mr.
Keene’s run, however, came to an abrupt halt one day when he was
met in the paddock by a Russian racing official, followed by Russian
chemist, complete with a basket of frogs. Some saliva was taken from Mr.
Keene's horse, and presumably force-fed to the frog, which then reportedly
behaved in a most un-frog-like way. Mr.
Keene's horse was duly declared "positive," and shortly
thereafter Mr. Keene left Russia and
returned to Kentucky and
to his family farm, Keeneland.
Classic
analytical chemistry based race testing as we know it started in
France
,
apparently before 1910. In
1935, Mr. William Woodward of Woodward Stakes fame, sent a Dr. Catlett, a
veterinarian and Dr. Charles Morgan, a chemist, from
Florida
to
France
to learn
the French drug testing techniques. They returned to
Florida
and set
up the first
US
drug
testing lab; later the New York Racing Commission opened a racing
chemistry laboratory on the10th floor of a building on
Chambers
Street
in
Manhattan
. In 1947
the professional association of racing chemists, the Association of
Official Racing Chemists [AORC] was formed.

Figure 4. The late Mr. Robert
Vessiny, Truesdail Labs, Tustin,
CA,
circa 2000. |
Robert
Vessiny's professional career began in 1941 at the NY Racing
Commission Laboratory on
Chambers
Street in
Manhattan and
continued until 2005, covering virtually the entire history of US
racehorse
testing which started about 1935
in
Florida
and
New
York under Dr. Charles Morgan. |
4. Can Drugs or Medications Influence
the Outcome of a Race?
|
Drugs
and medications can be used to influence the outcome of races in a number
of ways. Acute stimulant medication is the administration of a stimulant
substance to a horse shortly before post. Among the especially useful
agents in this area are the opiates, which have long been used in racing
horses, and also the amphetamine-like stimulants, and most especially
methylphenidate (Ritalin). All of these substances have been widely used,
the opiates likely for hundreds of years, and presumably particularly so
when testing for these agents was not available.
|
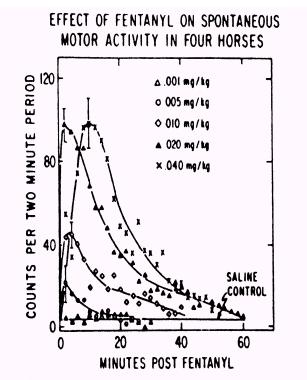
Figure 5. |
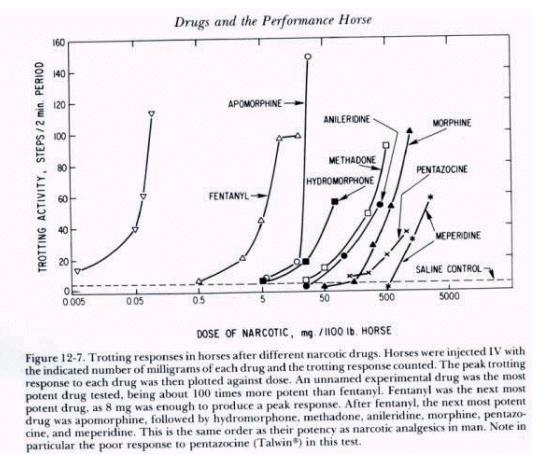
Figure 6.
Narcotic
drug family dose response curves
As drug
testing improved in sensitivity those individuals seeking an “opiate
edge” began to use the more potent and thus more difficult to detect
opiates. The unnamed but highly potent opiate at the far left of the above
family of opiate dose response curves (Fig. 6) is etorphine, or
“elephant juice.” Etorphine is one of the most potent opiates known
and at the time that this figure was published in “Drugs and the
Performance Horse” in 1981, there was no test available that could
detect it, which is the reason that this medication is unidentified in
this original paper. This
figure also shows, for one family of substances, the 10,000 fold range in
dose/potency from the least potent opiate tested on the right, meperidine,
at about a one gram/horse dose, to the very highly potent etorphine on the
far left, with 50 micrograms (50 millionths of a gram) producing an
equivalent pharmacological effect to one gram or more of meperidine. And,
of course, etorphine was also, in round figures, about 10,000 time more
difficult for the chemist to detect than the old standbys of morphine and
heroin, one of whose street names was “horse.” This great increase in
the potency of medications being used in horses set the stage for the
development of ELISA Testing, as we will discuss later.
As well as
being administered stimulants to win races, horses can also be medicated
to win by relaxing them and allowing the horse to run its best possible
race. The widely used tranquilizer acepromazine, and any number of related
or equivalent agents, have reportedly been used in this way.
Improving a horse’s “wind” by opening its airways through the use of
bronchodilators may also improve the performance of horse, and especially
a horse that is sub-clinically broncho-constricted. In this regard, the
best selling ELISA test at one time was a particularly sensitive and broad
scope bronchodilator test, the availability of which test abruptly stopped
the less than therapeutic use in racing horses of a bronchodilator called
terbutaline.

Figure 7.
Only triple dead heat on record |

Figure 8.
Grindstone wins the 1996
Kentucky Derby by a nose |
The difficulty with trying to scientifically demonstrate performance effects of drugs in small numbers of horses is that the drug needs to produce a positive performance effect of about the same magnitude as Secretariat’s win at Belmont
(Fig. 9) to meet the lowest level of statistical significance acceptable in science. This is a considerable experimental challenge; another way of looking at this is that successful horse trainers make far more subtle and discriminating judgments than most scientists, of which I think there is no doubt whatsoever. |

Figure 9. Secretariat wins at Belmont |
Veterinarians
certify horses as being sound in "wind and limb." Obviously,
medications that can affect these parameters and also the “attitude”
or “behavior” of a horse have the potential to affect both the
presentation of a horse and also, presumably, the results of the ultimate
performance analysis, the outcome of a race. By
the mid-1980s the use of highly potent drugs, and most
especially highly potent opiate type medications such as fentanyl (Sublimase)
and etorphine, and high potency bronchodilators to improve the racing
performance of horses had created considerable
problems for race horse testing and also for racing.
5. The Introduction of ELISA Testing, 1988
In
the mid-1980s, race testing was for all practical purposes dependent on a
primary screening technique called Thin Layer Chromatography (TLC). This technology has many useful qualities, being inexpensive
and fast, but it is not
particularly sensitive, and in the mid-1980s some horsemen were reportedly
using high potency narcotics, stimulants, bronchodilators and
tranquilizers with impunity. In 1985 we were requested (directed?) by the
then Kentucky State Racing Commission to "fix this problem." The
solution that we developed, ELISA testing for high potency drugs and
medications, is in place and widely used around the world today, and is
evidenced here in Lexington by a thriving commercial concern, Neogen Corp,
on Nandino Boulevard, employing 100 people and bringing in about US $50
million a year into Lexington (not all through ELISA tests – www.neogen.com/forensickits.htm).
The
term ELISA is an acronym that stands for Enzyme Linked ImmunoSorbent
Assay. Simply put, an ELISA test is a variant on the home pregnancy test
technology. It requires a drop of urine; it can be performed relatively
rapidly, it is/can be highly sensitive and can be read by eye. When ELISA
testing was first introduced, the problem was to keep the technology from
"putting down” too many trainers, especially in those jurisdictions
that had frozen “back samples.” Let me simply say that this was a
turbulent time for me professionally, but matters eventually settled down
and, as I indicated, ELISA testing is in many areas the backbone of drug
screening worldwide today.
|
The term ELISA is an acronym that stands for Enyme Linked ImmunoSorbent
Assay. Simply put, an ELISA test is a variant on the home pregnancy test technology. It requires a drop of urine; it can be performed relatively rapidly, it is/can be highly sensitive and can be read by eye. When ELISA testing was first introduced, the problem was to keep the technology from
"putting down” too many trainers, especially in those jurisdictions that had frozen “back samples”. Let me simply say that this was a turbulent time for me professionally, but matters eventually settled down and, as I indicated, ELISA testing is the backbone of drug screening worldwide today. |
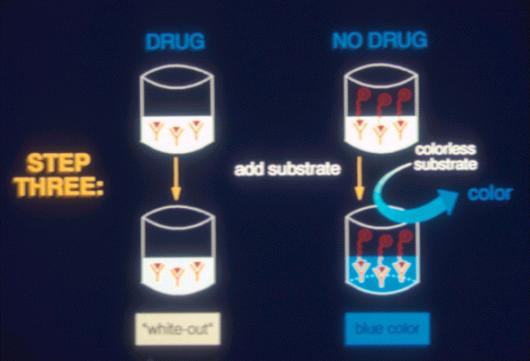
Figure 10. ELISA Testing |
This is a
96 well ELISA plate in which the full blue color of an ELISA negative has
been developed in most wells. The clear wells on the left hand side are
the “positive controls” containing calibration standards. All of the
other wells represent ELISA “negative” urine samples. A “track”
ELISA positive would show up as a clear well in the middle of the
blue samples, in laboratory jargon, a so called “whiteout,” or an
ELISA positive.
|
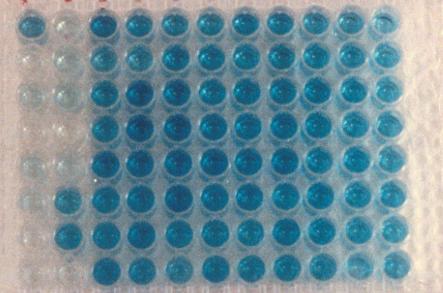
Figure 11. ELISA Test Results |
An
ELISA test will usually detect about 5 ng/ml (or 5 parts per billion) or
less of drug or drug metabolite in the sample.
Some tests are 10 fold more sensitive, detecting down to the high parts
per trillion. To put these figures in perspective, one part per billion is
one second in your life if you are 32 years old.
To put the matter of testing sensitivity into regulatory perspective, a
sure prescription for regulatory friction/problems is a therapeutic
medication (or a dietary or environmental substance) given at higher
(gram) doses to horses, excreted efficiently in urine, and being tested
for by an analyst with a highly sensitive ELISA test with no
thresholds/decision levels in place. In the absence of “thresholds” or
detection/decision limits in place, a sensitive ELISA test can become
basically a hunting tool/license for forensic chemists. Isoxsuprine,
an ARCI class 4 therapeutic medication, administered orally in gram
amounts per day, and excreted very efficiently and at very high
concentrations in equine urine, is a classic example.
Finally, we must always remember that an ELISA test simply binds to and
“sees” one side/surface of the medication molecule. Therefore, while
an ELISA “negative” is almost certainly a true negative, an ELISA test
will, by definition, interact to some extent with substances
other than the drug in question. As such, the rule with an ELISA
“positive” is that it can always be, by definition, a so-called
“false positive.” Which is, of course, why chemists invariably follow
ELISA screening with the much more chemically specific
technique of Mass Spectral “confirmation."
And again, on the other hand, within the performance limits of the assay,
an ELISA "negative" is virtually certainly a true
negative.
6. Mass Spectral Confirmation
While
ELISA screening/testing is fast and highly sensitive, it is, as set forth
above, far from specific. The second and absolutely critical and essential
step in the testing process is confirmatory testing, usually by Mass
Spectroscopy. In this step, the molecule is isolated and its precise mass
measured, and the molecule is also broken into a series of fragments. Both
the mass and relative proportions of these fragments (the fragmentation
pattern) are specific for the given drug, and are then matched with known
certified reference standards run through the Mass Spectrometer in
parallel with the test samples. As
such, a full scan mass spectrum, with appropriate matching controls, is
the "gold standard" in drug testing, and is considered
definitive evidence for the presence of the substance in the sample in
question. Additionally racing also almost always allows collection of an
independent or "split" or "referee" sample, which the
trainer can have analyzed independently [although sometimes more
independently than others!] to check for the presence of the substance in
question. Independent replication of the primary findings in the
"split" or "referee" analysis usually neutralizes any
substantial challenges in the area of the substance claimed identified and
for some classes of substances, namely therapeutic medications, dietary
and environmental substances, may well yield important mitigating or
exculpatory information.
|

Figure 12.
Dr. Lehner and the LC/MS/MS |
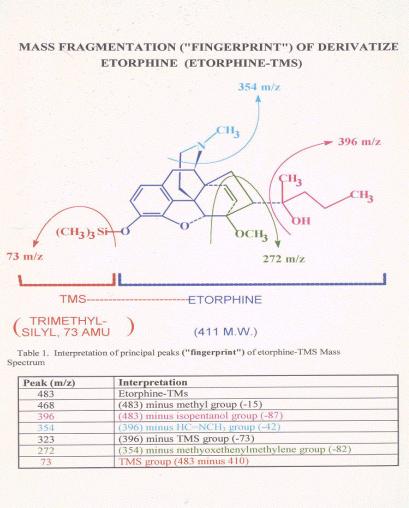
Figure 13. |
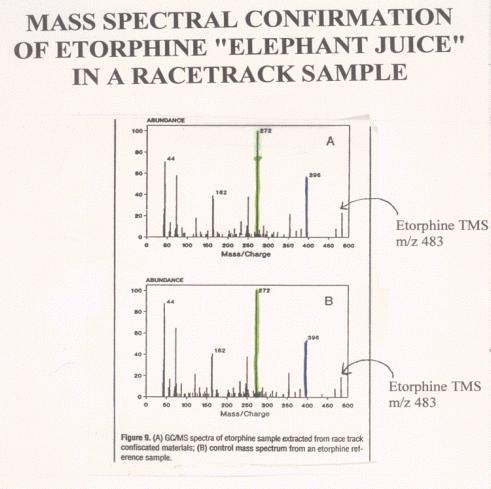
Figure 14. |
Comparison
of Mass Spectra of a post-race etorphine and an authentic standard. The
lower figure shows the mass spectrum of an authentic etorphine laboratory
standard. Note the molecular ion at mass 483, the base peak at mass 272
and the various other ions of the standard or control spectrum. Note the
very close correspondence of the standard or control mass spectrum with
the mass spectrum of the derivatized material recovered from the post-race
sample, indicating that the material recovered from the post race sample
is indistinguishable from authentic derivatized reference standard
etorphine.
7. Liquid Chromatography/Mass Spectrometry/
Mass Spectrometry (LC/MS/MS)
More recently an even higher sensitivity and
specificity technology has become widely available, namely, Liquid
Chromatography/Mass-Spectrometry/Mass-Spectrometry (LC/MS/MS).
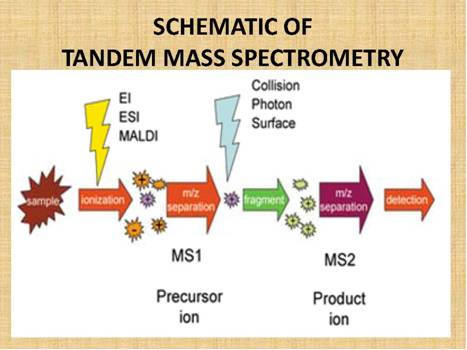
Figure
15.
Schematic
of MS/MS showing the relationship between the Mass Spectrometers and the
parent/precursor and product/daughter ions.
LC/MS/MS
technology allows the unequivocal identification and quantification of
substances down to low picogram per milliliter, that is, low part per
trillion concentrations, or at times even lower concentrations. The
technology is called LC because the separation technique, the liquid chromatographic
(LC) “feed” into the mass spectrometer, which expands the range of substances that can be
chromatographed and therefore analyzed.
The actual analysis takes place in a dual stage Mass Spectrometer
(MS/MS), linked
by a reaction/fragmentation chamber. The first Mass Spectrometer stage is
calibrated/set to identify a specific drug/molecular mass of interest, let
us say clenbuterol, which has a molecular mass of 277.19.
This drug molecule/mass then feeds into the reaction/fragmentation
chamber, where it is fragmented, and the molecular fragments are then fed
into the second Mass Spectrometer stage, where the product/daughter ion
fragments are identified. Figure
16 below shows the fragmentation of clenbuterol in our hands, showing how
the 277.19 mass clenbuterol molecule fragments to yield an intermediate of 259
and a final mass of 203, one
of several possible fragmentation sequences as set forth in (Fig. 16).
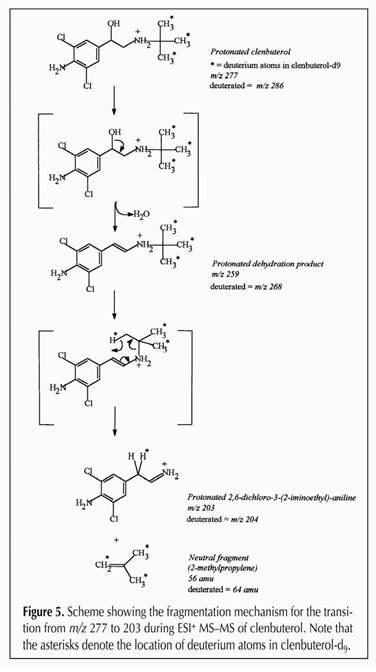
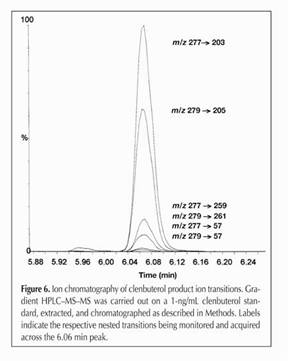
Figure 16.
The entire family of clenbuterol fragments, going from 277->203,
279->205, 277->259, 279->261, 277->57 and 277->57. As
we indicated, since each drug molecule has a specific starting mass,
and a specific fragmentation pattern and the relationship between
parent and daughter ions can be clearly identified, this is a highly
sensitive and highly specific analytical technique.
|
Figure
17. |
Finally,
given the very high sensitivity and specificity of this testing procedure,
it can be used to detect parts per trillion or picograms per ML of drug in
relatively small sample volumes, such as a one milliliter plasma sample,
as set forth in Reference 9 below.
This very high and continually increasing sensitivity of drug testing
techniques and instrumentation brings with it the problem of detecting
more and more minute traces of drugs, including therapeutic medications
used legitimately and appropriately to protect the health and welfare of
the horse, traces that are clearly pharmacologically and forensically
irrelevant.
As such, the extremely high sensitivity of current analytical
techniques has created
significant problems, and perhaps has made the
so called “zero-tolerance” concept or approach to equine
drug testing irrational and
irrelevant when
applied to therapeutic medications and dietary and environmental
substances.
8. Zero Tolerance Testing
“Zero
Tolerance” testing is not testing down to "Zero" molecules,
which no analytical chemist can yet accomplish, but rather testing to the
Limit of Detection (LOD) of the best available technology. While this may
be an entirely appropriate analytical approach to the regulation of
performance altering substances which have no place in racing, it is
absolutely not considered appropriate for therapeutic medications.
Therapeutic medications are substances used to maintain the health and
welfare of horses, and to arbitrarily change the sensitivity of testing
for these substances depending on either the whim of the chemist or
today's availability of improved testing technologies, is
entirely inappropriate, as we will see from review of the following basic
mathematics of drug/medication dosing and drug elimination.
9. Numbers of Medication Molecules:
Medication Dosing and Elimination
When you administer a dose of phenylbutazone to a
horse, you administer about the same number of phenylbutazone molecules as
there are stars in the known universe, that is about 6 followed by 21
zeros molecules. This is a very large number of molecules indeed.
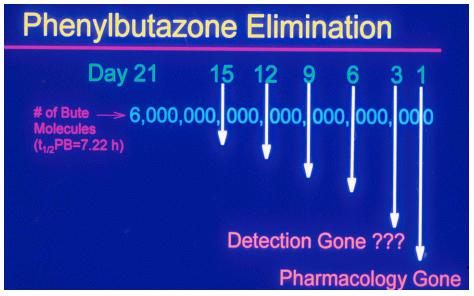
Figure 18.
The
horse will eliminate the bulk of this dose of phenylbutazone quite
rapidly. If phenylbutazone in the horse has a 7.22 hour half-life, 50% of
the drug will be eliminated by 7.22 hours after dosing, 75% by 14.44 hours
post dosing, 87.5 by about 21 hours post dosing, and 90% by 24
hours after dosing. At the end of day 1, when 90% of the drug is
eliminated, the pharmacological effect of the drug is, for all practical purposes,
gone, but there is still present in the horse the not inconsiderable
number of 6 followed by 20 zeros worth of phenylbutazone molecules. Every
day another 90% of the drug in the body of the horse will be eliminated,
and other zero drops off.
However, if
the chemist really wants to look, with current technology he or she can easily find traces of the
phenylbutazone or its metabolites for 14 days or more after administration, a time
post-administration that even the most conservative chemists and
regulators generally do not wish to pursue a medication identification.
However, the question now arises of when, precisely, should the chemist
stop pursuing these traces?
Or at what trace concentration should racing regulators cease being
concerned?
10. Thresholds, Including "No Effect Thresholds" (NETs)
The
answer to this question is simple; the chemist should stop pursuing these
traces precisely when he/she is instructed to stop. It is, however,
slightly more complicated to determine the exact point at which chemists should be instructed to cease and desist
their analytical
endeavors.
We approached this question experimentally in the
Maxwell
H.
Gluck
Equine
Research
Center during the second half of the
1990s. Simply put, we administered decreasing doses of local
anesthetics to horses until we saw no detectable local anesthetic effect,
which gave us the No Effect Dose for the drug, in this case a local
anesthetic. Then we measured the concentrations of the drug, actually a
recovered drug metabolite fragment, in the urine, and the concentrations
we came up with are, by experimental demonstration, not associated with
any pharmacological effect. These concentrations then become “No Effect
Thresholds” in urine for the specific therapeutic medication.
These No Effect Threshold [NET] concentrations can then be written
into the medication rules, and the chemist can then be advised not to test
below these now scientifically defined "no effect" regulatory
threshold concentrations.
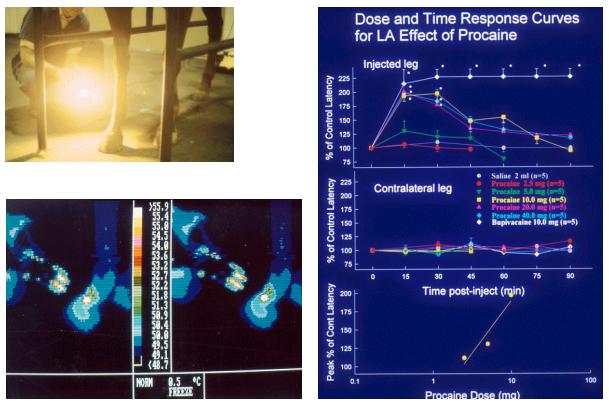
Figures 19-21. Equine response to
heat lamp stimuli
We
presented this scenario hypothetically in 1994-95, and then started the
actual research on regulatory thresholds. We were immediately vigorously
attacked from conservative quarters, at first anonymously and libelously.
In 1996 one of these libelous letters surfaced signed by Mrs. Donna Ewing
of the Illinois Hooved Animal Humane Society. The
University
of
Kentucky
"encouraged me,” shall we say, to sue, which I did. While
I eventually dropped the suit, its
filing had the desired effect
of silencing the complaining parties, who have not been heard from since
with regard to racing. More to the scientific point, we completed our
ongoing thresholds research and published it in the refereed scientific
literature. By the year 2000, the intellectual concept and more
importantly, the actual word "thresholds" became more or less
"safe" for a courageous racing administrator to allow past his
(or her) lips. Indeedby today December
2010, the concept of regulation by the use of specified concentration
“thresholds” in plasma or in urine is extremely well established, at
least in
North
America
.
In this
regard, is of interest to note that the concept/approach of "zero
tolerance" was, to some extent, officially voted out of favor and
“off the regulatory island” in one of the opening papers of the 13th
International Conference of Racing Analysts and Veterinarians (ICRAV 2000)
in Cambridge, England. In this
paper Professor Robert L. Smith addressed the concept of zero tolerance,
which he considered a "fading
illusion," and reviewed the events "which
are increasingly undermining the suitability of this approach."
In his words, "The zero
tolerance approach . . . is in essence an illusion in which the magician
is the racing chemist." He continued, "The
zero tolerance approach is both philosophically and pragmatically unsound.
. . . The goal for the future integrity of racing is to develop
"reporting values" for therapeutic substances based upon
rigorous analysis of their pharmacological and pharmacokinetic properties
and using an appropriate model.”
11. Withdrawal Time Guidelines
Let us now move
from the theoretical and illusionary concept of "zero tolerance"
to practical horsemen’s concerns. A “regulatory threshold” or a
“reporting level” is a concentration value (such as, for example 10 parts
per billion in urine) that has, in the larger scheme of things, little
actual reality for horsemen. This
is because a horseman or, for that matter, a chemist locked out of his/her
laboratory, cannot “see” 10 parts per billion of anything in horse
urine. What the horseman needs are clear, transparent “withdrawal time
guidelines,” i.e., guidelines as to when he/she should stop
administering the medication prior to post so that the blood or urine
"reading" comes in below the stipulated regulatory threshold,
whatever that particular threshold may be.
Establishing withdrawal time guidelines is considerably more difficult than
determining threshold concentrations. The only way to answer the
guidelines question is,
again, by actual experimental determination, followed by field
application. The specific medication product/formulation in question must
be defined/specified, and the formulation, dose, route, and duration and
number of administrations specified. The medication must be administered to
a significant number, hopefully at least 20 or more, Thoroughbred horses
in training, and the blood or urinary concentrations of the parent
medication or its principal urinary metabolite/analyte followed over a
period of time.
The laboratory performing the analyses should be appropriately accredited
(American Association of Laboratory Accreditation, A2LA), and have in
place a validated quantitative analytical method for the threshold substance at
concentrations down to concentrations significantly below the lowest
concentration of interest in
the experimental model/horses. (http://hbpa.org/resources/MedicationPolicy.pdf).
The data obtained must then be analyzed statistically, and fitted to a
defined mathematical distribution. One can then use this mathematical
distribution to advise horsemen that if they administer the drug following
X stipulation doses/days, and stop administration at Y hours prior to
post, there will be a Z probability of exceeding the regulatory threshold.
One of the things that everybody must understand is that if you administer
a medication to a horse at any time close to post, there is always a
finite mathematical probability of exceeding the threshold; all anybody
can do is estimate as accurately as possible the statistical probability
of exceeding the regulatory threshold, i.e., of incurring an
“overage”, and make sure that the risk of an inadvertent or
statistical “overage” is a risk that the horseman can live with.
This finite probability of a therapeutic medication overage is most likely
the reason that regulatory authorities are almost invariably reluctant to
be associated with “withdrawal time” guidelines. While a 1/1000 risk
of a “positive” may be an entirely acceptable risk for an individual
horseman running a small
number of horses, if the authority approves a given “withdrawal time,”
it immediately assumes responsibility for all 10-20,000 or more samples
tested in the jurisdiction, which increases the probability of a
statistical overage 10-20,000 fold, or more if the authority tests more
than 20,000 samples per year.
The first formal scientific approach to this question of determining a
regulatory threshold linked to a specific withdrawal time was undertaken
at the request of the Kentucky Horsemen's Benevolent and Protective
Association [KY HBPA] in the early 1980s. Responding
to a request from President Ed Flint of the Kentucky HBPA, we defined the
population distribution characteristics of furosemide in equine plasma
after its administration to 49 horses [Chay et al, Ref 11].
This work was soon published, and then picked up by a number of
racing states, starting with Oklahoma in about 1986.
Since then, the regulatory threshold for furosemide, adjusted
upwards to 100 ng/mL to allow for “field” variability and linked to a
1.010 urinary specific gravity value, has become the scientific basis for
the widely applied four hour furosemide rule in the United States, as set forth in the
ARCI medication thresholds/rules.
At the personal level, given the current state of knowledge, it is
at best extremely challenging to provide useful "withdrawal time
information" advice. The number of factors which affect the
withdrawal time is very large indeed, and in the absence of a defined
threshold ("Zero Tolerance" testing) it may be
little more that a guessing game. Whenever
I get a “withdrawal time” estimate request, I try to make these
uncertainties clear, and to also clearly communicate that any estimates
offered are nothing more that my best professional opinion, and my opinion is always qualified
with the statement that "there are no guarantees in life, and this
caveat most certainly includes
“withdrawal time” estimates."
The various factors that can affect "withdrawal times" are set
forth in some detail in Appendix #1, Thomas Tobin and Kent H. Stirling
(2009) Equine Drug Testing & Therapeutic Medication Regulation; 2009
Policy of the National Horsemen's Benevolent and Protective Association,
Inc. Wind Publications, 600 Overbrook Dr, Nicholasville, KY 40356, USA pp
170
12. Reference Standards
When
we administer a medication to a horse [or a human] what the chemist finds
in the urine is generally not the drug itself, but a chemically modified
[metabolized] form of the drug linked to a sugar molecule, glucuronic
acid. Figure 23 below sets
forth this process for lidocaine which is first hydroxylated by the horse
to give rise to 3-hydroxylidocaine. The hydroxylated lidocaine is
then linked to glucuronic acid to give the final metabolite,
3-hydroxylidocaine glucuronide. When
this resultant highly water-soluble glucuronide metabolite of
lidocaine enters the urine, it cannot be re-absorbed by the horse, and the
end result is a high urinary concentration of this glucuronide conjugated
metabolite of lidocaine, which is removed from the body of the horse the
next time the horse urinates.
And as an aside, where the metabolized drug goes after urination can be a matter of
some regulatory significance. If
the dose of drug administration to horses large, and the drug/metabolite
is excreted at high concentrations in the horse's urine, the horse will contaminate his stall
environment. It has been shown that a
"clean" horse put into the environmentally contaminated stall
can immediately go "positive" for the medication in question,
creating an interesting regulatory circumstance, and demonstrating another compelling
argument in favor of regulatory thresholds for therapeutic medications.
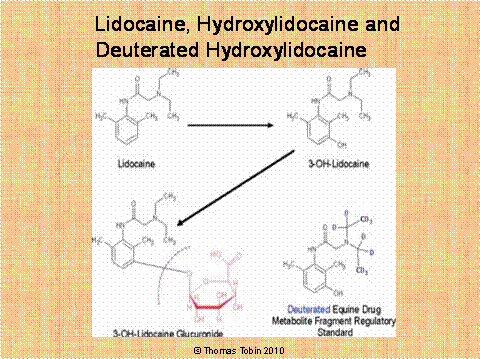
Figure
23.
Lidocaine is first
hydroxylated by the horse to give rise to 3-hydroxylidocaine; this
hydroxylated lidocaine is then linked to glucuronic acid to give the final
highly water soluble metabolite, 3-hydroxylidocaine glucuronide.
Analyzing this sample, the chemist first hydrolyses off the
glucuronic acid, and then recovers the 3-hydroxylidocaine metabolite
fragment from the urine sample and quantifies it. To correctly perform
this analysis requires a certified reference standard for
3-hydroxylidocaine and a deuterated internal standard in which 10 of the
hydrogen atoms in 3-hydroxylidocaine have been replaced with 10 deuterium
atoms, as set forth in Appendix II
To quantify
the amount of this 3-hydroxylidocaine glucuronide metabolite in a urine
sample, the chemist needs a certified reference standard for
3-hydroxylidocaine, and also what is called a deuterated version of
3-hydroxylidocaine, which serves as an internal or "loss check"
reference standard throughout the analytical procedure.
At the time that we started this work there was no source for these
reference standards and internal standards, so we began creating a line of
certified reference standards and internal standards for equine
therapeutic medications. Creation
of these standards is not a trivial process, and the synthetic scheme set
forth below is that generated by our colleagues Dr. Rodney Eisenberg and
Dr. Julio Gutierrez for the synthesis of the required 3-hydroxylidocaine
certified reference standard and internal standard, as set forth in
APPENDIX III.
13. Medication Rules
More
than forty years ago, when the
Kentucky
medication rule was first being formulated (even before I came to Lexington)
there were no thresholds or regulatory limits anywhere. Indeed, there were
very few, if any, quantitative analytical methods applied to racing. Under
these circumstances the Kentucky
medication rule was clear, simple, effective and highly practical. You
could not run your horse on stimulants, depressants, local anesthetics,
tranquilizers or narcotic analgesics, the classic performance altering
substances. However, the use of substances that were perceived as
therapeutic was permitted, with the goal of protecting the health and
welfare of the horse. This Kentucky
rule was well fitted to the regulatory technology then available, and
indeed is, I understand, close to the rule currently applied in human
athletics. At that time, this very practical rule had been
in place in Kentucky for at least 30 years and, to the best of my
knowledge, served the horses and horsemen of Kentucky well.
14. The Current Racing Medication Testing
Consortium (RMTC) & Association of
Racing
Commissioners International (ARCI) Rule
As of December
2010 the following is a summary of the RMTC/ARCI model rules referring to
thresholds for therapeutic medications. Additionally, one must keep in
mind that at any given time individual states may have in place thresholds
that at times differ from and/or extend the RMTC/ARCI model rules. Many
of these individual state thresholds are presented in the National HBPA
booklet, Appendix #1, but given the rate of change of medication rules,
for definitive information with respect to any individual state, the most
up to date version of the individual state’ medication rules should
always be consulted.
The current Association of Racing Commissioners International model
rule includes the thresholds presented below (Table 2.). Again, for
definitive information the Association of Racing Commissioners
International should be consulted. See the ARCI website http://www.arci.com.
NONSTEROIDAL
ANTI-INFLAMMATORY MEDICATIONS:
Phenylbutazone
- 2 micrograms per
milliliter;
Flunixin - 20 nanograms
per milliliter;
Ketoprofen - 10 nanograms
per milliliter.
Phenylbutazone [subthreshold] - 0.5
micrograms per milliliter; |
|
FUROSEMIDE:
Furosemide
- 100 nanograms
per milliliter, urinary specific gravity < 1,010 |
|
ANTI-ULCER
MEDICATIONS
Cimetidine - up to 24
hours before post
Omeprazole - up to 24 hours
before post
Ranitidine - up to
24 hours before post |
|
ENVIRONMENTAL
SUBSTANCES
Caffeine
- 100 ng of caffeine per ml in plasma or serum |
|
ANDROGENIC-ANABOLIC
STEROIDS:
a)
16β-hydroxystanozolol (metabolite of stanozolol (Winstrol)) - 1
ng/ml in urine for all horses regardless of sex;
(b) Boldenone (Equipoise® is the undecylenate ester of boldenone)
in male horses other than geldings - 15 ng/ml in urine. No boldenone
shall be permitted in geldings or female horses.
(c) Nandrolone (Durabolin® is the phenylpropionate ester and
Deca-Durabolin® is the decanoate ester)
(A) In geldings - 1 ng/ml in urine
(B) In fillies and mares - 1 ng/ml in urine
(d) Testosterone
(A) In geldings - 20 ng/ml in urine
(B) In fillies and mares - 55 ng/ml in urine |
|
ALKALINIZING
SUBSTANCES
The
threshold for TCO2 is 37.0 millimoles per liter of plasma/serum or a
base excess level of 10.0 millimoles, and;
The decision level to be used for the regulation of TCO2 is 37.0
millimoles per liter of plasma/serum plus the measurement
uncertainty of the laboratory analyzing the sample, or a base excess
level of 10.4 millimoles per liter of plasma/serum. |
Table 2. RMTC/ARCI
model threshold rules
Finally, as of this writing, the RMTC appears committed to
developing regulatory thresholds in plasma for all of the therapeutic
medications listed in Table 1. This is a significant regulatory advance based on
recent technological and regulatory developments set forth in this medication regulation overview.
13. Further Reading
1/
www.thomastobin.com
2/ Thomas Tobin. Drugs and the Performance Horse by Thomas Tobin, 463
pages, Charles C. Thomas, Springfield, Illinois, 1981.
3/ Thomas Tobin and Kent H. Stirling.
Equine
Drug Testing & Therapeutic Medication Regulation:
2009 Policy of the National Horsemen's Benevolent and Protective
Association, Inc., Wind Publications, Nicholasville, Kentucky,
2009,
170 pages.
4/ Tobin T, Mundy GD,
Stanley
SD, Sams RA, Crone D (eds):
Testing for Therapeutic Medications, Environmental and Dietary Substances
in Racing Horses, Proceedings of Workshop, Lexington,
KY, 220 pages, 1995. [KY Ag Exp
Sta #95-14-058]
5/ The Association of
Racing Commissioners International (ARCI) Uniform Classification System
for Foreign Substances: http://www.arci.com/druglisting.pdf
6/ Tobin T, Harkins JD, Sams RA: Testing for therapeutic medications:
Analytical/ pharmacological relationships and the need for
“limitations” on the sensitivity of testing for certain agents. J Vet
Pharm Therap, 22:220-233. 1999. [KY Ag Exp Sta #98-14-134].
7/ Smith R.L “The zero tolerance approach to doping control in horse
racing: a fading illusion.” Proceedings of the 13th International
Conference of Racing Analysts and Veterinarians (ICRAV)
Cambridge
,
United Kingdom
, p 9-14, 2000.
8/
Tobin
T, Watt
DS, Kwiatkowski
S, Tai
HH, Blake
JW, McDonald
J, Prange
CA, Wie
S.
Non-isotopic immunoassay drug tests in racing horses: a review of their
application to pre- and post-race testing, drug quantitation, and human
drug testing. Res
Commun Chem Pathol Pharmacol. 1988 Dec;62(3):371-95.
9/ Neogen ELISA tests: www.neogen.com/forensickits.htm
10/
Lehner A.F.; Harkins
J.D.; Karpiesiuk
W.; Woods
W.E.; Robinson
N.E.; Dirikolu
L.; Fisher
M.; Tobin
T; .Clenbuterol in the Horse:
Confirmation and Quantitation of Serum Clenbuterol by LC/MS/MS after
Oral and Intratracheal Administration; Journal of Analytical
Toxicology, Volume 25, Number 4, May/June 2001 , pp.
280-287(8)
11/ Chay
S, Woods
WE, Rowse
K, Nugent
TE, Blake
JW, Tobin
T. 1983The pharmacology of furosemide in the horse. V. Pharmacokinetics and blood
levels of furosemide after intravenous administration Drug
Metab Dispos. May-Jun;11(3):226-31.
12/ Thomas Tobin, et al. Furosemide in the Horse. Wind
Publications, Nicholasville, Kentucky, 2000.
Appendix #1
Table of Contents from Equine
Drug Testing & Therapeutic Medication Regulation:
2009 Policy of the National Horsemen's Benevolent and Protective
Association, Inc.
by Thomas Tobin and Kent H. Stirling
Wind Publications, Nicholasville, KY
40356
, USA
pp 170
Appendix #2
SYNTHESIS
OF 3-HYDROXYLIDOCAINE
& DEUTERATED 3-HYDROXYLIDOCAINE
The
synthesis of 3-hydroxylidocaine started with commercially available
2,6-dimethylaniline 1, which
required 4 steps to produce 3-hydroxylidocaine.
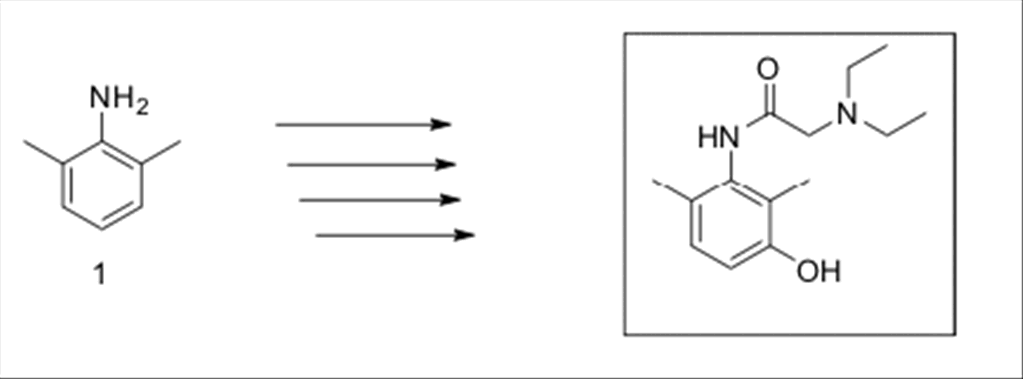
Scheme
1:
Synthesis of
3-hydroxylidocaine.
Synthesis
of 3-hydroxylidocaine-d10
starts with the same starting material 1,
above, and then follows steps
similar to those used to produce 3-hydroxylidocaine..
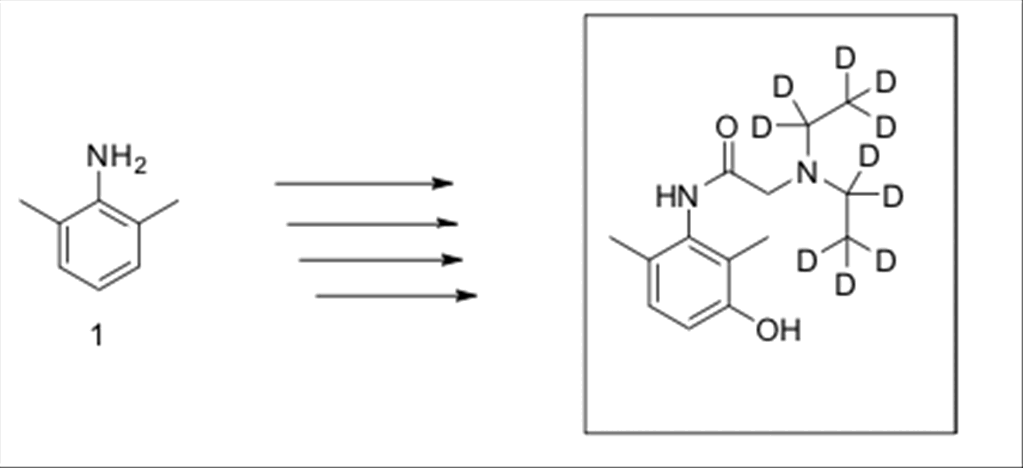
Scheme
2:
Synthesis of 3-hydroxylidocaine-d10.
Appendix #3
|
EQUINE
MEDICATION AND METABOLITE REFERENCE
STANDARDS AND STABLE ISOTOPE STANDARDS
Julio Gutierrez, Wojtek Karpiesiuk, Gabrielle Herrensmith, Elizabeth
Armstrong,
Charlie Hughes, Job Tharappel, and Thomas Tobin.
The
Maxwell
H.
Gluck
Equine
Research
Center
,
Department of Veterinary Science,
University
of
Kentucky
,
Lexington
,
KY
40546
Rodney Eisenberg.
Frontier Biopharm,
P.O.
Box 614
,
Richmond
,
KY
40476
Brent Mayer and Emilie Stanley.
Neogen Corp.,
Nandino Boulevard
,
Lexington
,
KY
40511
The equine medication metabolite standards and stable isotope reference
standards listed here are being synthesized by a team of
scientists, starting with Dr. Wojciech Karpiesiuk in the Gluck
Equine Research Center, more recently in cooperation with Dr.
Rodney Eisenberg and more recently again with Dr. Julio Gutierrez
and Ms. Gabrielle Herrensmith, whose synthetic methods have been
guided by the analytical skills of Dr. Andreas Lehner, Dr. Job
Tharappel and Mr. Charlie Hughes. These syntheses have been an
ongoing project, starting in the mid-nineties with the need to
synthesize reference standards for unique equine drug metabolites
and metabolite fragments. More recently, there has been an
increasing need for high-quality certified reference standards and
stable isotope internal standards for use in quantitative equine
medication regulation, or more simply, to allow the regulatory
application of thresholds for therapeutic medications.
The
University
of
Kentucky
and Frontier Biopharm, in cooperation with Neogen Corp., are
working to provide the horse race testing community with certified
quantitative analytical reference standards for equine therapeutic
medications that will satisfy the ISO-17025 and ISO-34
requirements for quantitative measurements, and related reference
materials which are increasingly required for equine forensic
testing. These reference standards will meet industry requirements
for chemical identity, spectroscopic and chemical purity, and the
levels of residual volatile solvents, water and inorganic, in
accordance with accepted ISO analytical standards.
Synthesis of these standards has been made possible by ongoing
research support from a large number of Horsemen’s Benevolent
and Protective Association’s, starting with the Florida HBPA in
the mid-90s, and since then including the National Horsemen’s
Benevolent and Protective Association and the Alabama, Arizona,
Arkansas, Canada, Charles Town West Virginia, Florida, Iowa,
Indiana, Kentucky, Louisiana, Michigan, Minnesota, Nebraska, Ohio,
Oklahoma, Ontario Canada, Oregon, Pennsylvania, Tampa Bay Downs
Florida, Texas, Washington State, and West Virginia Horsemen’s
Benevolent and Protective Associations, the Kentucky Equine Drug
Research Council, and the Kentucky Racing Commission, the Kentucky
Horse Racing Authority and the Kentucky Science and Engineering
Foundation, Grant agreement KSEF 148-502-05-160 with the Kentucky
Science and Technology Corporation, and this support is gratefully
acknowledged, along with the ongoing support of the Director and
Faculty of the Maxwell H. Gluck Equine Research Center and the
University of Kentucky College of Agriculture.
|
Standards
Available or in
Synthesis
|
|
No.
|
Parent Medication
|
Regulatory Analyte or
Deuterated Internal Standard
|
|
1
|
Acepromazine
|
2-(1-hydroxyethyl)
promazine sulfoxide (D4)
(deuterated metabolite)
|
|
2
|
Acepromazine
|
2-(1-hydroxyethyl)
promazine sulfoxide
(metabolite)
|
|
3
|
Bupivacaine
|
3-Hydroxybupivacaine
(metabolite)
|
|
4
|
Clenbuterol
|
Clenbuterol
(D9 )
(deuterated standard)
|
|
5
|
Detomidine
|
Carboxydetomidine
(metabolite)
|
|
6
|
Detomidine
|
Carboxydetomidine
(D4)
(deuterated metabolite)
|
|
7
|
Detomidine
|
Hydroxydetomidine
(metabolite)
|
|
8
|
Detomidine
|
Hydroxydetomidine
(D4)
(deuterated metabolite)
|
|
9
|
Furosemide
|
Furosemide
(D5)
(deuterated standard)
|
|
10
|
Flunixin
|
Flunixin
(D3)
(deuterated standard)
|
|
11
|
Guafenesin
|
Guafenesin
(D4)
(deuterated standard )
|
|
12
|
Ketoprofen
|
Ketoprofen
(D3)
(deuterated standard )
|
|
13
|
Lidocaine
|
3-Hydroxylidocaine
(metabolite)
|
|
14
|
Mazindol
|
2-(2-Aminoethyl)-3-(4-Chlorophenyl3-Hydroxy-2,3-dihydro-isoindol-1-one
(metabolite)
|
|
15
|
Mepivacaine
|
3-Hydroxymepivacaine
(metabolite)
|
|
16
|
Methocarbamol
|
Methocarbamol
(D4)
(deuterated standard )
|
|
17
|
Modafinil
acid
|
Modafinil
acid
(metabolite)
|
|
18
|
Phenylbutazone
|
Phenylbutazone
(D9 )
(deuterated standard)
|
|
19
|
Procaine
|
Procaine
(D10)
(deuterated
standard )
|
|
20
|
Promazine
|
3-Hydroxypromazine
(metabolite)
|
|
21
|
Promethazine
|
Promethazine
sulfoxide
(metabolite)
|
|
22
|
Propanolol
|
4-Hydroxypropanolol
(metabolite)
|
|
Click
here to see Certificates of Analysis as a PDF document.
Metabolites are filed under the name of the parent
medication. zA
thru M z
N
thru Z
Wait a moment for PDF to load after clicking.
If you do not have the Acrobat PDF Reader you may download
Acrobat here.
|
|
|
|
Also Synthesized or
Synthesis in Progress
|
|
No.
|
Parent
Medication
|
Regulatory
Analyte or
Deuterated Internal Standard
|
|
1
|
Acepromazine
|
Acepromazine
Sulfoxide
(metabolite)
|
|
2
|
Amitraz
|
N-2,4-Dimethylphenyl-N’-methyl-formamidine
(D6)
(deuterated standard)
|
|
3
|
Chlorpromazine
|
7-Hydroxychlorpromazine
(metabolite)
|
|
4
|
Clenbuterol
|
1-(4-Amino-3,5-Dichlorophenyl)ethane-1,2-diol
(metabolite )
|
|
5
|
Clenbuterol
|
2-(2-)4-Amino-3,5-
dicholorophenyl)-2-hydroxyethylamino)-2-methyl-propan-1-ol
(metabolite)
|
|
6
|
Colterol
and Bitolterol
|
3-0-Methoxycolterol
(metabolite)
|
|
7
|
Fluphenazine
|
7-Hydroxyfluphenazine
(metabolite)
|
|
8
|
Guanabenz
|
Hydroxyguanabenz
(metabolite)
|
|
9
|
Lidocaine
|
3-hydroxylidocaine
(D10)
(deuterated standard )
|
|
10
|
Mazindol
|
2-(2-Aminoethyl)-3-(4-chlorophenyl)-3-hydroxy-2,3-dihydro-isoindol-1-one
(metabolite)
|
|
11
|
Mepivacaine
|
3-Hydroxymepivacaine
(D3)
(deuterated standard )
|
|
12
|
Propiomazine
|
2-(1-Hydroxypropyl)promethazinesulfoxide
(metabolite)
|
|
13
|
Propionylpromazine
|
2-(1-Hydroxypropyl)promazine
sulfoxide
(metabolite)
|
|
14
|
Pyrilamine
|
Pyrilamine
(D4)
(deuterated standard)
|
|
15
|
Ropivacaine
|
3-Hydroxyropivacaine
(metabolite)
|
|
16
|
Ropivacaine
|
4-Hydroxyropivacaine
(metabolite)
|
|
17
|
Selegiline
|
Desmethylselegiline
(metabolite)
|
|
18
|
Tramadol
|
Desmethyltramadol
(metabolite)
|
|
Appendix #4
Association
of Racing Commissioners International “Drug Positives”
ARCI
Drug Postive Rulings From
8/1/2004
Thru
8/1/2005
4
ACEPROMAZINE
1 ACETYLSALICYLIC ACID (ASPIRIN)
10 ALBUTEROL
4 AMINOREX
4 AMPHETAMINE
1 BENZOCAINE
9 BENZOYLECGONINE
1 BETAMETHASONE
1 BOLDENONE
1 BUMETANIDE
1 BUSPIRONE
1 BUTORPHANOL
16 CAFFEINE
3 CAFFEINE, THEOPHYLLINE
1 CAFFEINE, THEOBROMINE, THEOPHYLLINE
1 CARPROFEN
1 CELECOXIB
3 CIMETIDINE
26 CLENBUTEROL
6 CROMOLYN
1 DANTROLENE
1 DESMETHYLPYRILAMINE
1 DESMETHYLPYRLAMINE
1 DETOMIDINE
45 DEXAMETHASONE
1 DEXTRORPHAN
14 DICLOFENAC
20 DIMETHYLSULFOXIDE
1 DIPHENHYDRAMINE
1 DIPRENOPHINE
3 DORMOSEDAN
2 EPHEDRINE
1 ERGONOVINE
3 EXCESS TCO2
1 FEXOFENADINE
1 FLUMETHASONE
85 FLUNIXIN
4 FLUNIXIN/PHENYLBUTAZONE
1 FLUPHENAZINE
25 FUROSEMIDE
3 GUAIFENESIN
6 GUANABENZ
1 HALOPERIDOL
2 HYDROCORTISONE AND MEVIPICAINE
1 HYDROMORPHONE
1 HYDROXYDANTROLENE
2 HYDROXYDETOMIDINE
2 HYDROXYETHYL PROMAZINE SULFOXIDE
1 HYDROXYLIDOCAINE
1 HYDROXYMEPIVACAINE
1 IPRATROPIUM
4 IPRATROPIUM BROMIDE
1 ISOFLUPREDONE
2 ISOXSUPRINE
6 KETOPROFEN
18 KETOROLAC
10 LASIX
2 LIDOCAINE
3 MEPIVACAINE
7 METHAMPHETAMINE
24 METHOCARBAMOL
16 METHYLPREDNISOLONE
2 MORPHINE
9 NAPROXEN
1 NAPROXEN POSITIVE
2 NAPROXENTHE
1 NAQUASONE
1 NORPSEUDOEPHEDRINE DESMETHYLPYRILAMINE
1 O-DESMETHYLPYRILAMINE AND NORPSEUDOEPHEDRINE
2 PENTAZOCINE
2 PENTOXYFYLLINE
1 PERINDOPRIL
1 PHENYLBUTAZONE
4 PHENYOXYPHEN
1 PIRBUTEROL
3 POLYETHYLENEGLYCOL
15 PROCAINE
2 PROPANTHELINE
1 PROPRANOLOL
1 PSEUDOEPHEDRINE
1 PSEUDOEPHEDRINE And NORPSEUDOEPHEDRINE
1 PSEUDOEPHEDRINE AND NORPSEUDOEPHEDRINE AND DESMETHYLPYRILAMI
2 PYRILAMINE
8 RANITIDINE
1 SALICYLIC ACID
4 SALIX
1 TERBUTALINE POSTV
5 THEOPHYLLINE
47 TOTAL CARBON DIOXIDE (TCO2)
3 TRIAMCINOLONE
4 TRICHLORMETHIAZIDE
4 TRIMETHOPRIM
4 TRIPELENNAMINE
23 UNKNOWN
1 VENTIPULMIN SYRUP
DETAILS OF THE FIELD AND MEDICATION REGULATION
National Horsemen’s Benevolent and Protective Association, Inc. Proposed National Policy on Drug Testing and Therapeutic Medication. J Eq Vet Sci 23(1): 4-5, 18-40, 2003.
http://hbpa.org/resources/MedicationPolicy.pdf
Published as Kentucky Agricultural Experiment Station Article
#_____ with the approval of the Dean and Director, College of Agriculture and the Kentucky Agriculture Experiment Station.
Publication #359 From the Equine Pharmacology, Therapeutics and Toxicology Program of the Maxwell H. Gluck Equine Research Center, University of Kentucky, Lexington, KY 40546-0099.
Supported by the National, Alabama, Arizona, Arkansas, Charles Town WV, Florida, Iowa, Kentucky, Louisiana, Michigan, Minnesota, Nebraska, Ohio, Oklahoma, Oregon, Pennsylvania, Tampa Bay Downs, Texas, Washington State, West Virginia, Ontario, and Canada Horsemen's Benevolent and Protective Associations.
|


























